At What Temperature Does Water Freeze
Water is one of the most essential substances on Earth, and its freezing point is a fundamental aspect of our daily lives. The temperature at which water freezes is a critical parameter in various fields, including science, engineering, and everyday activities. In this article, we will delve into the world of water freezing, exploring the factors that influence its freezing point and the implications of this phenomenon.
Key Points
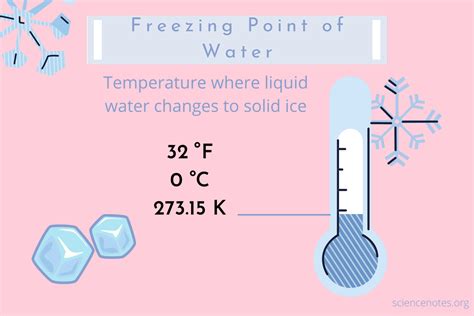
- The freezing point of water is 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F) at standard atmospheric pressure.
- The freezing point of water can be affected by factors such as pressure, purity, and the presence of impurities.
- The freezing process of water is a complex phenomenon that involves the formation of ice crystals and the release of latent heat.
- Understanding the freezing point of water is crucial in various applications, including refrigeration, cryogenics, and water treatment.
- The study of water freezing has led to significant advances in our understanding of thermodynamics, phase transitions, and materials science.
The Freezing Point of Water

The freezing point of water is defined as the temperature at which water changes state from a liquid to a solid. At standard atmospheric pressure, the freezing point of water is 0°C or 32°F. This temperature is also known as the melting point of ice, as it is the temperature at which ice melts to form water.
Factors Influencing the Freezing Point of Water
The freezing point of water can be influenced by various factors, including pressure, purity, and the presence of impurities. For example, the freezing point of water decreases with increasing pressure, a phenomenon known as the “pressure melting point.” This means that water can remain in a liquid state at temperatures below 0°C if the pressure is high enough.
Additionally, the presence of impurities in water can affect its freezing point. For instance, saltwater has a lower freezing point than freshwater, which is why seawater typically freezes at a temperature around -1.8°C. This phenomenon is known as "freezing point depression" and is an important consideration in various applications, including water treatment and desalination.
| Factor | Effect on Freezing Point |
|---|---|
| Pressure | Decreases with increasing pressure |
| Purity | Affects freezing point due to impurities |
| Impurities | Can lower or raise freezing point depending on type and concentration |

The Freezing Process of Water

The freezing process of water is a complex phenomenon that involves the formation of ice crystals and the release of latent heat. When water is cooled to its freezing point, the molecules begin to slow down and come together to form a crystalline structure. This process is known as nucleation, and it is the first step in the formation of ice.
As the water continues to cool, the ice crystals grow and become more ordered, releasing latent heat in the process. This heat is transferred to the surrounding environment, causing the temperature to rise. The freezing process of water is an exothermic reaction, meaning that it releases heat energy into the surroundings.
Implications of Water Freezing
Understanding the freezing point of water has significant implications for various applications, including refrigeration, cryogenics, and water treatment. For example, the freezing point of water is critical in the design of refrigeration systems, where it is used to cool fluids and gases to extremely low temperatures.
In addition, the study of water freezing has led to significant advances in our understanding of thermodynamics, phase transitions, and materials science. By exploring the complex phenomena that govern the freezing process of water, researchers can gain insights into the underlying mechanisms that govern these complex systems.
What is the freezing point of water at standard atmospheric pressure?
+The freezing point of water at standard atmospheric pressure is 0°C or 32°F.
How does pressure affect the freezing point of water?
+The freezing point of water decreases with increasing pressure, a phenomenon known as the "pressure melting point."
What is the effect of impurities on the freezing point of water?
+The presence of impurities in water can affect its freezing point, with some impurities lowering the freezing point and others raising it.
In conclusion, the freezing point of water is a complex phenomenon that is influenced by various factors, including pressure, purity, and the presence of impurities. Understanding the freezing point of water has significant implications for various applications, including refrigeration, cryogenics, and water treatment. By exploring the complex phenomena that govern the freezing process of water, researchers can gain insights into the underlying mechanisms that govern these complex systems.



