Enantiomer Vs Diastereomer

The realm of stereochemistry is a fascinating aspect of organic chemistry, where the three-dimensional arrangement of atoms in molecules plays a crucial role in determining their properties and behavior. Two key concepts in this field are enantiomers and diastereomers, which refer to different types of stereoisomers that arise due to the presence of chiral centers in molecules. Understanding the differences between enantiomers and diastereomers is essential for chemists and researchers working in various fields, including pharmaceuticals, materials science, and biotechnology.
Introduction to Stereoisomers
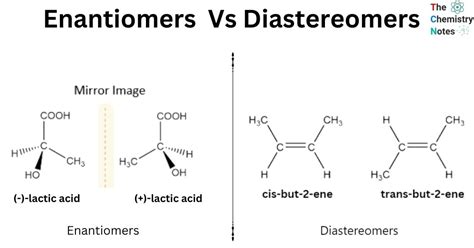
Stereoisomers are molecules that have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional arrangement of their atoms. This difference in arrangement can lead to distinct physical and chemical properties, making stereoisomers an important area of study in chemistry. Stereoisomers can be classified into two main categories: enantiomers and diastereomers. Enantiomers are non-superimposable mirror images of each other, while diastereomers are stereoisomers that are not mirror images of each other.
Key Points
- Enantiomers are non-superimposable mirror images of each other.
- Diastereomers are stereoisomers that are not mirror images of each other.
- The presence of chiral centers in molecules gives rise to stereoisomers.
- Stereoisomers can have distinct physical and chemical properties.
- Understanding stereoisomers is essential in various fields, including pharmaceuticals and biotechnology.
Enantiomers: Non-Superimposable Mirror Images
Enantiomers are pairs of molecules that are non-superimposable mirror images of each other. This means that if one enantiomer is reflected in a mirror, the resulting image will be identical to the other enantiomer. Enantiomers have the same physical and chemical properties, except for their ability to rotate plane-polarized light in opposite directions. This property is known as optical activity, and it is a characteristic feature of enantiomers. Enantiomers can be distinguished from each other using various methods, including optical rotation, circular dichroism, and chromatographic techniques.
| Property | Enantiomer 1 | Enantiomer 2 |
|---|---|---|
| Molecular Formula | C4H10 | C4H10 |
| Physical Properties | Same | Same |
| Optical Activity | +10° | -10° |

Diastereomers: Non-Mirror Image Stereoisomers
Diastereomers are stereoisomers that are not mirror images of each other. Unlike enantiomers, diastereomers can have different physical and chemical properties, such as melting points, boiling points, and reactivity. Diastereomers can also exhibit different biological activities, which is important in pharmaceutical applications. Diastereomers can be classified into different types, including cis-trans isomers, syn-anti isomers, and anomers. Each type of diastereomer has its unique characteristics and properties, making them an interesting area of study in stereochemistry.
Importance of Stereochemistry in Real-World Applications

Stereochemistry plays a vital role in various real-world applications, including pharmaceuticals, materials science, and biotechnology. The understanding of enantiomers and diastereomers is essential in the development of new drugs, where the correct stereoisomer can have a significant impact on the efficacy and safety of the drug. In materials science, the study of stereochemistry can help in the design of new materials with unique properties, such as chiral catalysts and optically active polymers. In biotechnology, the understanding of stereochemistry is crucial in the development of new biological agents, such as enzymes and antibodies.
In conclusion, the study of enantiomers and diastereomers is a fascinating area of stereochemistry, with significant implications in various fields. Understanding the differences between these two types of stereoisomers is essential for chemists and researchers working in pharmaceuticals, materials science, and biotechnology. By recognizing the importance of stereochemistry, we can develop new molecules with unique properties and applications, ultimately contributing to the advancement of science and technology.
What is the difference between enantiomers and diastereomers?
+Enantiomers are non-superimposable mirror images of each other, while diastereomers are stereoisomers that are not mirror images of each other.
Why is stereochemistry important in pharmaceutical applications?
+Stereochemistry is important in pharmaceutical applications because the correct stereoisomer can have a significant impact on the efficacy and safety of a drug.
What are some common methods for distinguishing between enantiomers?
+Common methods for distinguishing between enantiomers include optical rotation, circular dichroism, and chromatographic techniques.