Face Centered Cubic

The Face Centered Cubic (FCC) structure is a common crystal lattice arrangement found in many metals and alloys. It is characterized by a cubic unit cell with atoms located at the corners of the cube and at the center of each of the six faces. This arrangement is also known as the cubic close-packed (CCP) structure. The FCC structure is significant in materials science due to its unique properties, such as high density, high thermal conductivity, and excellent ductility, making it suitable for various industrial applications.
Crystal Structure and Properties
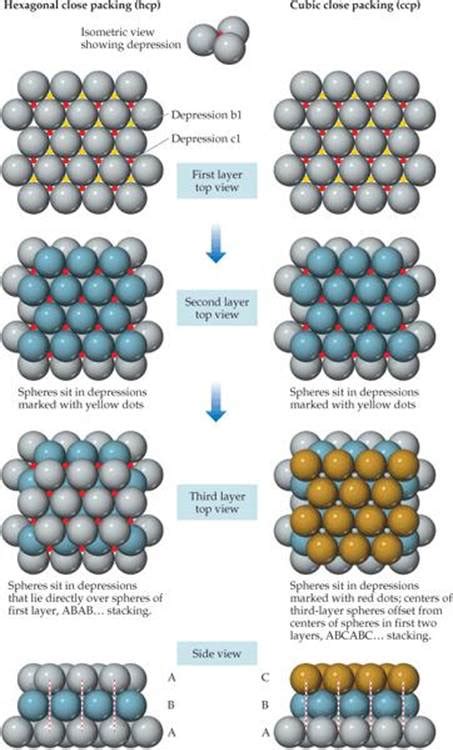
The FCC structure can be visualized as a series of layers, with each layer consisting of a hexagonal arrangement of atoms. The layers are stacked in an ABC sequence, where each atom in one layer is positioned directly above the center of a triangle formed by three atoms in the layer below. This arrangement results in a high packing density, with approximately 74% of the available space being occupied by the atoms. The high packing density and the resulting strong bonding between the atoms contribute to the excellent mechanical properties of FCC materials.
Atomic Radius and Lattice Parameter
In an FCC lattice, the relationship between the atomic radius ® and the lattice parameter (a) is given by the equation r = a / (2 * sqrt(2)). This equation can be used to calculate the lattice parameter from the known atomic radius of the material. The lattice parameter is an important parameter in determining the physical properties of the material, such as its density, thermal expansion, and elastic constants.
| Material | Atomic Radius (nm) | Lattice Parameter (nm) |
|---|---|---|
| Copper | 0.128 | 0.3615 |
| Aluminum | 0.143 | 0.4049 |
| Gold | 0.144 | 0.4078 |

Materials with Face Centered Cubic Structure

Many metals and alloys exhibit the FCC structure, including copper, aluminum, gold, silver, and nickel. These materials are widely used in various industries due to their excellent mechanical, thermal, and electrical properties. For example, copper is used extensively in electrical wiring and circuits due to its high electrical conductivity, while aluminum is used in the aerospace industry due to its high strength-to-weight ratio.
Applications and Properties
The FCC structure is also found in some ceramic materials, such as cesium chloride (CsCl) and barium titanate (BaTiO3). These materials have unique properties, such as high ionic conductivity and piezoelectricity, making them suitable for applications in fuel cells, sensors, and actuators. The FCC structure is also being researched for its potential applications in nanotechnology, where the unique properties of materials at the nanoscale can be exploited for innovative devices and systems.
Key Points
- The Face Centered Cubic (FCC) structure is a common crystal lattice arrangement found in many metals and alloys.
- The FCC structure is characterized by a cubic unit cell with atoms located at the corners of the cube and at the center of each of the six faces.
- The FCC structure has a high packing density, with approximately 74% of the available space being occupied by the atoms.
- The high packing density and the resulting strong bonding between the atoms contribute to the excellent mechanical properties of FCC materials.
- Many metals and alloys exhibit the FCC structure, including copper, aluminum, gold, silver, and nickel.
Conclusion and Future Directions
In conclusion, the Face Centered Cubic structure is an important crystal lattice arrangement with unique properties and applications. Its high packing density, high thermal conductivity, and excellent ductility make it suitable for various industrial applications. Further research is needed to fully understand the properties and behavior of FCC materials, particularly at the nanoscale, where new and innovative applications are being developed. The study of FCC materials is an active area of research, with new discoveries and advancements being made regularly.
What is the difference between the Face Centered Cubic (FCC) and Body Centered Cubic (BCC) structures?
+The main difference between the FCC and BCC structures is the location of the atoms within the unit cell. In the FCC structure, atoms are located at the corners of the cube and at the center of each of the six faces, while in the BCC structure, atoms are located at the corners of the cube and at the center of the cube.
Which materials exhibit the Face Centered Cubic structure?
+Many metals and alloys exhibit the FCC structure, including copper, aluminum, gold, silver, and nickel. Some ceramic materials, such as cesium chloride (CsCl) and barium titanate (BaTiO3), also exhibit the FCC structure.
What are the advantages of the Face Centered Cubic structure?
+The FCC structure has a high packing density, which results in strong bonding between the atoms and excellent mechanical properties. It also has high thermal conductivity and excellent ductility, making it suitable for various industrial applications.



