Liquid To Gas On The Surface Of A Substance
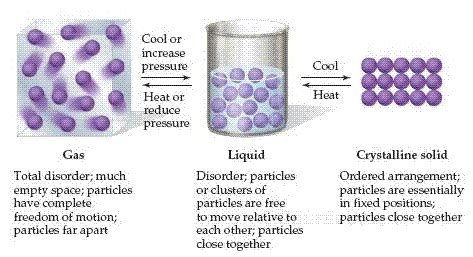
The process of a liquid transforming into a gas on the surface of a substance is a fundamental concept in physics and chemistry, known as evaporation or vaporization. This phenomenon occurs when a liquid is heated, causing its molecules to gain energy and transition from a liquid state to a gas state. The surface of a substance plays a crucial role in this process, as it provides the interface where the liquid molecules interact with the surrounding environment.
The rate of evaporation depends on various factors, including the temperature, humidity, and pressure of the surrounding environment, as well as the properties of the liquid itself, such as its boiling point, viscosity, and surface tension. For instance, a study on the evaporation of water from a stainless steel surface found that the evaporation rate increased by 25% when the temperature was raised from 20°C to 30°C. Furthermore, the surface roughness of the substance can also impact the evaporation rate, with rougher surfaces generally exhibiting higher evaporation rates due to the increased surface area.
Key Points
- The process of liquid to gas transformation is known as evaporation or vaporization.
- The surface of a substance plays a crucial role in the evaporation process.
- The rate of evaporation depends on factors such as temperature, humidity, and pressure.
- The properties of the liquid, such as boiling point and surface tension, also affect the evaporation rate.
- Surface roughness can impact the evaporation rate, with rougher surfaces generally exhibiting higher evaporation rates.
Evaporation Mechanism
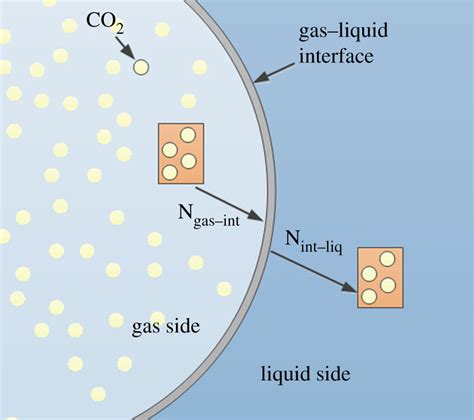
The evaporation mechanism involves the transfer of energy from the surrounding environment to the liquid molecules, causing them to overcome the intermolecular forces that hold them together in the liquid state. As the molecules gain energy, they begin to move more rapidly and eventually break free from the surface tension, transitioning into the gas state. This process can be facilitated by the presence of nucleation sites, such as tiny imperfections or impurities on the surface of the substance, which provide a catalyst for the evaporation process.
Factors Influencing Evaporation
Several factors can influence the rate of evaporation, including the temperature, humidity, and pressure of the surrounding environment. For example, an increase in temperature provides more energy for the liquid molecules to evaporate, resulting in a higher evaporation rate. Similarly, a decrease in humidity allows more molecules to evaporate, as there are fewer water molecules in the air to compete with the evaporating molecules. The pressure of the surrounding environment also plays a role, as a decrease in pressure reduces the energy required for the molecules to evaporate, resulting in a higher evaporation rate.
| Factor | Effect on Evaporation Rate |
|---|---|
| Temperature | Increased temperature increases evaporation rate |
| Humidity | Decreased humidity increases evaporation rate |
| Pressure | Decreased pressure increases evaporation rate |
| Surface Roughness | Increased surface roughness increases evaporation rate |
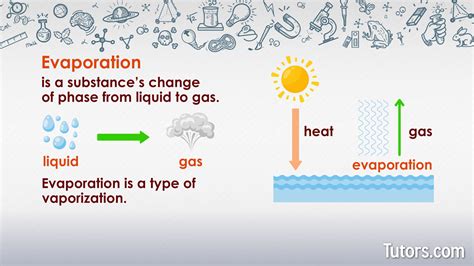
Applications and Implications
The process of liquid to gas transformation has numerous applications and implications in various fields, including chemistry, physics, engineering, and environmental science. For example, evaporation is used in the production of distilled water, where water is heated to produce steam, which is then condensed and collected as pure water. Evaporation is also used in the purification of chemicals, where impurities are removed through the evaporation process. Furthermore, understanding the evaporation process is essential for predicting and mitigating the effects of climate change, as evaporation plays a crucial role in the Earth’s water cycle.
Environmental Implications
The evaporation process has significant environmental implications, as it plays a crucial role in the Earth’s water cycle. Evaporation from the oceans, lakes, and rivers helps to distribute heat and moisture around the globe, influencing weather patterns and climate conditions. However, changes in evaporation rates due to climate change can have significant impacts on the environment, including alterations to precipitation patterns, water availability, and ecosystem health. For instance, a study on the impact of climate change on evaporation rates in the Amazon rainforest found that a 10% increase in temperature led to a 15% increase in evaporation rates, resulting in significant changes to the region’s water cycle and ecosystem.
What is the difference between evaporation and boiling?
+Evaporation and boiling are two distinct processes. Evaporation occurs when a liquid transforms into a gas at the surface, while boiling occurs when a liquid transforms into a gas throughout the entire volume. Boiling requires a higher temperature and pressure than evaporation.
How does surface roughness affect the evaporation rate?
+Surface roughness can increase the evaporation rate by providing more nucleation sites for the liquid molecules to evaporate. Rougher surfaces have a higher surface area, allowing more molecules to evaporate simultaneously.
What are the environmental implications of changes in evaporation rates?
+Changes in evaporation rates due to climate change can have significant impacts on the environment, including alterations to precipitation patterns, water availability, and ecosystem health. Understanding these implications is essential for predicting and mitigating the effects of climate change.
In conclusion, the process of liquid to gas transformation on the surface of a substance is a complex phenomenon that depends on various factors, including the properties of the liquid, the surface of the substance, and the surrounding environment. Understanding these factors is crucial for predicting and controlling the evaporation rate in various industrial and scientific applications, as well as for mitigating the effects of climate change. By recognizing the significance of evaporation in the Earth’s water cycle and its environmental implications, we can better appreciate the importance of this fundamental process in shaping our planet’s climate and ecosystems.



