Periodic Table Image
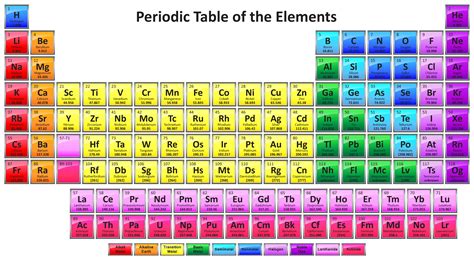
The periodic table is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families.
Structure of the Periodic Table

The periodic table is structured in a way that elements with similar properties are placed in the same group, while elements with similar electron configurations are placed in the same period. The periodic table is divided into several blocks, including the s-block, p-block, d-block, and f-block, which are based on the electron configuration of the elements.
Blocks of the Periodic Table
The s-block consists of the elements in groups 1 and 2, which are the alkali metals and alkaline earth metals, respectively. The p-block consists of the elements in groups 13 to 18, which are the boron group, carbon group, nitrogen group, chalcogens, halogens, and noble gases. The d-block consists of the elements in groups 3 to 12, which are the transition metals. The f-block consists of the elements in the lanthanide and actinide series, which are also known as the inner transition metals.
| Block | Groups | Elements |
|---|---|---|
| s-block | 1 and 2 | Alkali metals and alkaline earth metals |
| p-block | 13 to 18 | Boron group, carbon group, nitrogen group, chalcogens, halogens, and noble gases |
| d-block | 3 to 12 | Transition metals |
| f-block | Lanthanide and actinide series | Inner transition metals |

Key Points
- The periodic table is a tabular display of the known chemical elements, organized by their atomic number and electron configuration.
- The elements are grouped into rows called periods and columns called groups or families.
- The periodic table is divided into several blocks, including the s-block, p-block, d-block, and f-block.
- The blocks are based on the electron configuration of the elements and are used to predict the properties and behavior of the elements.
- The periodic table is a powerful tool for understanding the properties and behavior of elements and has played a crucial role in the development of chemistry and physics.
One of the key features of the periodic table is the periodic trends, which are the patterns of change in the properties of the elements as you move from one element to another. These trends include the trends in atomic radius, electronegativity, and ionization energy, among others.
Periodic Trends
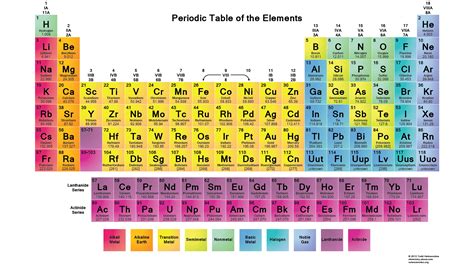
The periodic trends are due to the changes in the electron configuration of the elements as you move from one element to another. The trends can be explained by the periodic law, which states that the properties of the elements are periodic functions of their atomic numbers.
Atomic Radius Trend
The atomic radius trend is the trend in the size of the atoms as you move from one element to another. The atomic radius generally decreases as you move from left to right across a period and increases as you move down a group.
| Period | Atomic Radius Trend |
|---|---|
| Decreases from left to right | Increases from top to bottom |
What is the periodic table?
+The periodic table is a tabular display of the known chemical elements, organized by their atomic number and electron configuration.
What are the blocks of the periodic table?
+The blocks of the periodic table are the s-block, p-block, d-block, and f-block, which are based on the electron configuration of the elements.
What are the periodic trends?
+The periodic trends are the patterns of change in the properties of the elements as you move from one element to another, including the trends in atomic radius, electronegativity, and ionization energy.
In conclusion, the periodic table is a powerful tool for understanding the properties and behavior of elements, and it has played a crucial role in the development of chemistry and physics. The periodic trends, including the trends in atomic radius, electronegativity, and ionization energy, are due to the changes in the electron configuration of the elements as you move from one element to another.