What Is Freezing Point In Degrees Fahrenheit
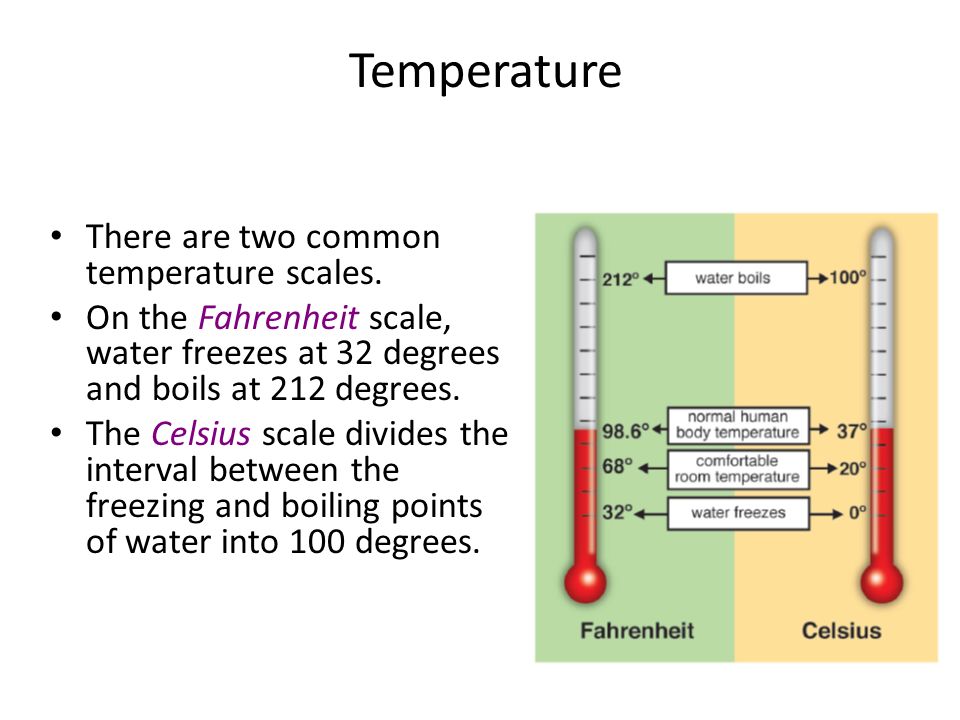
The freezing point is the temperature at which a liquid changes state to become a solid. This phenomenon occurs when the molecules of a substance slow down and come together in a crystalline structure, releasing heat energy into the surroundings. In the context of water, which is the most common reference point for discussions about freezing points, this temperature is 32 degrees Fahrenheit (°F) at standard atmospheric pressure.
Understanding Freezing Point

The freezing point is a fundamental physical constant that is crucial in various scientific and everyday applications. It is defined as the temperature at which the liquid and solid phases of a substance are in equilibrium. For water, 32 °F is the temperature at which water can exist in both its liquid and solid (ice) states, assuming standard atmospheric conditions. This temperature is not only important for understanding natural phenomena like the formation of ice in lakes and rivers but also for numerous industrial and culinary processes.
Factors Influencing Freezing Point
The freezing point of a substance can be influenced by several factors, including pressure, the presence of impurities (which can lead to freezing point depression), and the specific characteristics of the substance itself. For instance, seawater has a lower freezing point than pure water due to the dissolved salts it contains. This adjustment in freezing point is critical for understanding oceanic phenomena and the formation of sea ice. Furthermore, the freezing point can be altered under different pressure conditions, a principle utilized in technologies such as refrigeration systems.
| Substance | Freezing Point (°F) |
|---|---|
| Water | 32 |
| Seawater (approximate) | 28.4 |
| Methanol | -143.7 |
| Ethanol | -173.2 |

In scientific research and industrial applications, the accurate determination of a substance's freezing point is crucial for characterizing its properties and predicting its behavior under different conditions. Techniques such as differential scanning calorimetry (DSC) are commonly used to measure freezing points with high precision, providing valuable data for both theoretical models and practical applications.
Applications and Implications

The freezing point has significant implications in various fields. In biology, understanding the freezing points of water and other solvents is critical for preserving biological samples and understanding the survival strategies of organisms in cold environments. In food science, knowledge of freezing points is essential for the preservation of food quality and safety, influencing the texture, nutritional value, and microbial safety of frozen foods.
Environmental Considerations
From an environmental perspective, the freezing point of water plays a pivotal role in climate science. Changes in sea ice coverage, which are influenced by the freezing point of seawater, have profound effects on global climate patterns, ocean currents, and marine ecosystems. Furthermore, the study of freezing points in relation to glaciers and ice caps helps scientists understand historical climate conditions and predict future changes.
Key Points
- The freezing point of water is 32 °F at standard atmospheric pressure.
- Impurities and pressure changes can alter the freezing point of a substance.
- Understanding freezing points is crucial for various scientific, industrial, and culinary applications.
- Accurate measurement of freezing points is essential for characterizing substances and predicting their behavior.
- The freezing point has significant environmental implications, including effects on climate patterns and marine ecosystems.
In conclusion, the freezing point, specifically 32 degrees Fahrenheit for water, is a fundamental concept with far-reaching implications across multiple disciplines. Its understanding and application are vital for advancing our knowledge of natural phenomena, improving industrial processes, and addressing environmental challenges.
What is the freezing point of water in degrees Fahrenheit?
+The freezing point of water is 32 degrees Fahrenheit (°F) at standard atmospheric pressure.
How do impurities affect the freezing point of a substance?
+Impurities can lower the freezing point of a substance, a phenomenon known as freezing point depression. This is why seawater, which contains dissolved salts, has a lower freezing point than pure water.
What are some applications of understanding freezing points?
+Understanding freezing points is crucial for various applications, including food preservation, climate science, and the development of cooling systems. It is also essential for predicting the behavior of substances under different conditions and for characterizing their properties.