What Is Simple Diffusion

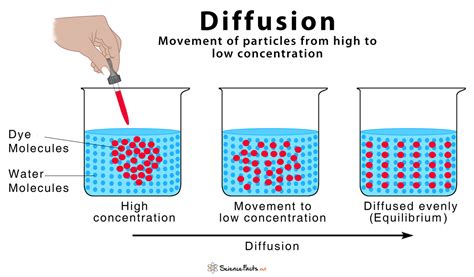
Simple diffusion is a fundamental concept in physics and biology, describing the process by which particles or molecules move from an area of higher concentration to an area of lower concentration, resulting in uniform distribution. This movement is driven by the kinetic energy of the particles and does not require energy input from the surroundings. Simple diffusion is a passive transport mechanism, meaning it does not need the assistance of proteins or other cellular structures to facilitate the movement of substances across cell membranes or through other barriers.
The rate of simple diffusion depends on several factors, including the concentration gradient of the substance, the size and shape of the particles, the viscosity of the medium through which the particles are moving, and the temperature. A higher concentration gradient, smaller particle size, lower viscosity, and higher temperature all contribute to an increased rate of diffusion. Simple diffusion plays a crucial role in various biological processes, such as the exchange of oxygen and carbon dioxide between the lungs and the bloodstream, the absorption of nutrients from the digestive system into the bloodstream, and the elimination of waste products from the body.
Key Points
- Simple diffusion is a passive transport process that moves particles from an area of higher concentration to an area of lower concentration.
- The rate of simple diffusion is influenced by the concentration gradient, particle size, medium viscosity, and temperature.
- Simple diffusion is essential for various biological functions, including gas exchange, nutrient absorption, and waste removal.
- This process does not require energy input from the surroundings, relying on the kinetic energy of the particles.
- Understanding simple diffusion is critical for appreciating how substances move within living organisms and across cell membranes.
Factors Influencing Simple Diffusion

The efficiency and rate of simple diffusion are significantly affected by several key factors. The concentration gradient, which is the difference in concentration of the substance across the membrane or barrier, is a primary driver of diffusion. A steeper concentration gradient results in faster diffusion, as particles move more quickly from areas of higher concentration to areas of lower concentration. The size and shape of the diffusing particles also play a crucial role; smaller particles can diffuse more rapidly than larger ones due to their greater mobility and ability to pass through smaller spaces.
The viscosity of the medium through which the particles are diffusing is another critical factor. A less viscous medium allows particles to move more freely and rapidly, whereas a highly viscous medium slows down the diffusion process. Temperature affects the kinetic energy of the particles; higher temperatures provide more energy for particle movement, thereby increasing the rate of diffusion. Lastly, the surface area available for diffusion can influence the rate at which substances are exchanged or distributed. A larger surface area can facilitate faster diffusion by providing more pathways for particle movement.
Biological Applications of Simple Diffusion
Simple diffusion has numerous biological applications, particularly in the context of cellular physiology and the exchange of substances within organisms. One of the most critical applications is in the respiratory system, where oxygen from the inhaled air diffuses into the blood through the alveoli in the lungs, while carbon dioxide, a waste product of metabolism, diffuses out of the blood and into the exhaled air. This process is vital for the oxygenation of tissues and the removal of carbon dioxide.
In the digestive system, simple diffusion plays a role in the absorption of certain nutrients from the gastrointestinal tract into the bloodstream. Although many nutrients require facilitated diffusion or active transport to be absorbed, simple diffusion can contribute to the absorption of smaller, lipid-soluble molecules. Additionally, simple diffusion is involved in the elimination of waste products by the kidneys, where waste substances diffuse from the blood into the urine, which is then excreted from the body.
| Factor | Influence on Diffusion Rate |
|---|---|
| Concentration Gradient | Directly proportional; steeper gradient increases diffusion rate |
| Particle Size | Inversely proportional; smaller particles diffuse more rapidly |
| Medium Viscosity | Inversely proportional; lower viscosity increases diffusion rate |
| Temperature | Directly proportional; higher temperature increases diffusion rate |
| Surface Area | Directly proportional; larger surface area increases diffusion rate |
Conclusion and Future Perspectives

In conclusion, simple diffusion is a vital process that underpins numerous biological functions, from the exchange of gases in the lungs to the absorption of nutrients in the digestive system. Its role in maintaining homeostasis and facilitating the removal of waste products cannot be overstated. As our understanding of biological systems and the factors influencing simple diffusion continues to evolve, so too will our ability to apply this knowledge in medical and environmental contexts. Future research directions may include exploring how alterations in diffusion rates impact disease states and developing novel therapeutic strategies that exploit or modify diffusion processes to enhance substance delivery or removal.
What is the primary driving force behind simple diffusion?
+The primary driving force behind simple diffusion is the concentration gradient, which is the difference in concentration of a substance across a membrane or barrier. Particles naturally move from areas of higher concentration to areas of lower concentration until equilibrium is reached.
How does temperature affect the rate of simple diffusion?
+Temperature has a direct influence on the rate of simple diffusion. An increase in temperature provides more kinetic energy to the particles, allowing them to move more rapidly and increasing the rate of diffusion. Conversely, a decrease in temperature slows down particle movement, reducing the diffusion rate.
What role does simple diffusion play in the respiratory system?
+In the respiratory system, simple diffusion is crucial for the exchange of oxygen and carbon dioxide between the lungs and the bloodstream. Oxygen diffuses from the inhaled air into the blood, while carbon dioxide diffuses out of the blood and into the exhaled air, facilitating gas exchange and maintaining proper oxygenation of tissues and removal of carbon dioxide.